Blowing in the wind: forecasting pathogens to improve phoma and stem canker control
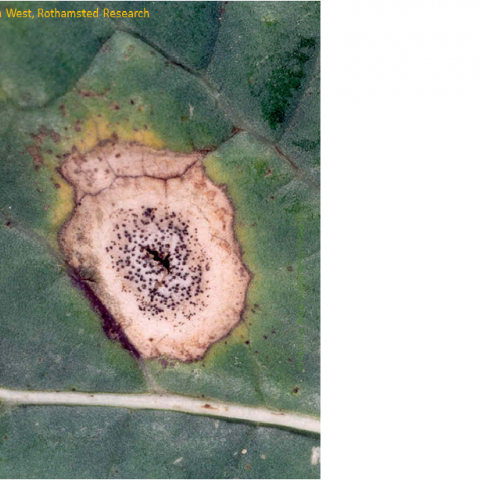
For those living in parts of the south and east of England, the switch from summer to autumn came very suddenly three weeks into September, when unusually dry and warm weather changed to a deluge of rain which has continued up to now (mid-October). Places further north and west had more rain during the summer and these differences in weather have a dramatic effect on the timing of some of our most important crop diseases. One of them, I’ve studied for many years, is phoma stem canker, which initially infects oilseed rape leaves to cause phoma leaf spot.
The cycle starts with stem infections or stem canker on the previous crop. After harvest, the fungus continues to develop on stem debris, initially seen as fruiting bodies called pycnidia, which release asexually-produced spores that are splashed short distances by rain. Later, sexual structures also emerge on the stem debris, resembling a tiny puffball about ¼-½ mm in diameter and these need a period of growth and maturation while, inside them, many bags containing eight ascospores develop. Their development, like all life as we know it, needs moisture and warmth. A dry period in summer causes the debris to desiccate and all maturation of fruiting bodies is put on hold until new rain or dew moistens the fungus allowing another period of development.
Even when it is wet, a cool period will slow the development down but eventually, as we get into autumn and the debris is made wet almost every day by rain or dew, the bags of mature ascospores swell with water, pressurising the inside of the tiny structure until one of the bags of spores is forced to the small pore at the top of the fruiting body and bursts, blasting one or a few spores at a time to be shot out into faster-moving air. At the mercy of the wind, many of these will not end up being deposited on a suitable host plant but a small number will make it to a nearby field of oilseed rape (OSR), land on the leaves and if conditions remain moist, they will germinate to start infecting the leaf. A tiny number of ascospores might even blow tens of miles, possibly more, to start a new infection but most will go less than a few hundred metres. The ascospores of the two species that cause stem canker, Leptosphaeria maculans and L. biglobosa, typically have six cells per spore and not all will germinate first time – some wait for another wet period in case the first were not successful.
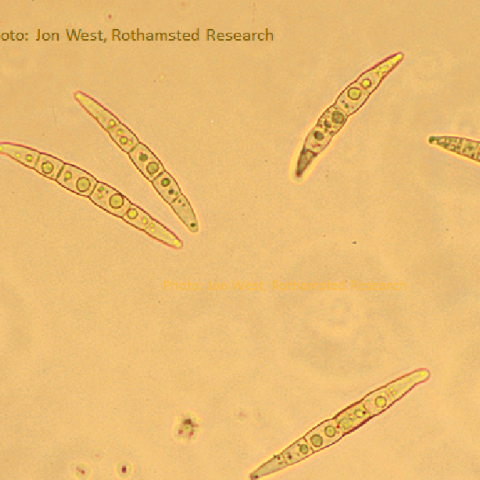
If the OSR variety is susceptible, the resulting phoma leaf spot continues the disease cycle with the fungus growing down the leaf stalk (petiole) to the stem. It’s a race really, with the future of that individual fungus hanging on whether it can grow down the vascular tissue of the leaf and make it to the stem before the leaf ages and is shed. There are factors affecting this, such as the age of the leaf when it was infected, plant or canopy density and light quality, fertiliser regime (which affects petiole length) and of course – fungicide use. If the fungus makes it to the stem, it then waits until the following spring, when it develops the visible and damaging stem canker. Another novel way to stop the fungus reaching the stem has been done in New Zealand on turnip rape, which is to graze sheep on the field, which eat back the leaves and the plant re-sprouts in the spring without the fungus.
In the UK, only fairly severe stem cankers (rotting more than half the cross-section of the stem in any one position by the end of flowering) cause significant yield loss due to disrupting water uptake to cause early stress and senescence and sometimes lodging. Warmer countries suffer significant yield loss from less-severe cankers due to a greater demand for water as it is lost from the upper parts of the plant.
Unfortunately, once the fungus has made it to the stem, there are no fungicides that can eradicate it. So, control relies on a combination of good host resistance and for conventional farmers, preventative fungicides. The most severe cankers tend to be caused by the earliest leaf infections that make it to the stem. So, timing fungicides right to prevent these has been the reason for using weather-based forecasting. In this case, summer temperature and wetness at different locations, are used to predict the maturation and release of spores from those fruiting bodies and the time when 10% of plants will have phoma leaf spot as this is a key time to apply fungicides to slow down already-incubating infections and prevent new infections. This should help – at least until new, untreated leaves unfold – hopefully with enough time passed that infections of those later leaves will not lead to severe cankers. Occasionally in ‘early seasons’, a second autumn fungicide might be applied. This year’s forecast is on the AHDB website.
In addition to phoma, a different disease – light leaf spot, caused by Pyrenopeziza brassicae, has a different type of forecast because its spores are already being released through late summer and early autumn when the new OSR crops are emerging. Although it has a long symptomless incubation period, causing symptoms to remain invisible until nearly Christmas, decisions on which fungicide regime to use is less about timing but more about the predicted severity of the disease. To do that, a mathematical model uses information on the previous season’s disease severity, the average summer temperature and the average expected winter rainfall (still to come) to give an initial forecast in early October. This is updated in the spring, using actual winter rainfall for each location to fine-tune the forecast, which could help with decisions on whether to do an additional fungicide spray to protect flowers and pods.

Whether you use fungicides or not, factors to reduce these diseases include using varieties with good disease resistance (few if any current varieties are completely resistant), promotion of good establishment and early vigour and use of crop rotation to maximise separation from sources of those pesky airborne spores.
To summarise:
- Choosing resistant varieties (see AHDB recommended lists) reduces dependence on fungicides and give more flexibility. For example, an old variety, ES Astrid, had very good polygenic resistance, which means the stem canker phase was rarely severe and often didn’t affect yield.
- It always helps to use more diverse or longer rotations at farm scale.
- Good early vigour can make plants more resistant to canker. This can be achieved with an early sowing and a small early application of fertiliser. It can also be worth topping up on micronutrients, especially sulphur to boost resistance.
- Check the AHDB disease forecast to help with spray timing decisions for both phoma leaf spot and light leaf spot.
Jon West is a senior research scientist at Rothmasted Research.
“I’m from the Isle of Sheppey in north Kent originally but have been in Harpenden for over 20 years, where I am a senior research scientist at Rothamsted Research, working on applied crop protection projects. I have always been interested in biology and farming and obtained a BSc in Biology from Royal Holloway, London in 1990 and a PhD in Plant Pathology at Reading in 1994. My recent work has focused on the biology and control of fungal diseases, which is important for food security and reducing the carbon footprint of farming. This work includes measurement of plant disease resistance, early detection of diseases and dispersal of spores and pollen. I am on committees of the Association of Applied Biologists and the British Crop Production Council diseases working group and I am a member of the British Society for Plant Pathology. Previously, I was a member of the European Food Safety Authority plant health panel (2015-18). I have an active interest in communicating science to students and the public.”



